Cambridge Raman Imaging (“CRI” or the “Company”) announces today it will coordinate a project selected to receive a €3.3 million grant in the European Innovation Council’s (EIC) Transition call.
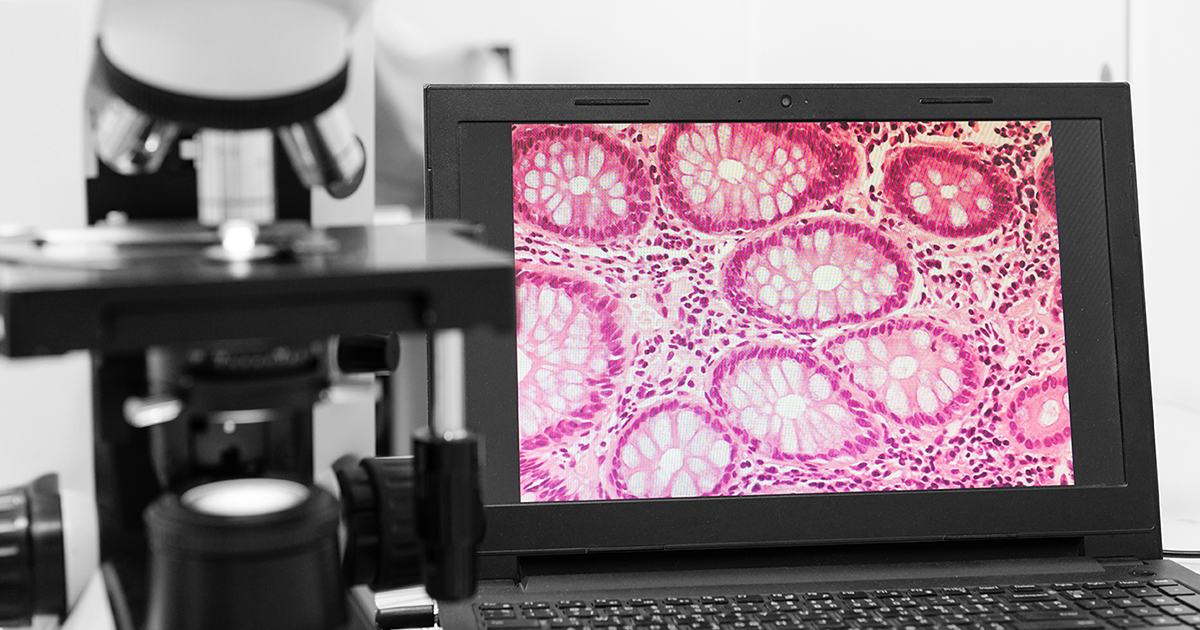
The project, called CHARM, aims to develop a medical device based on high-speed, low-cost Raman digital imaging technology and artificial intelligence to transform cancer diagnosis and treatment. The technology will analyse the molecular composition of patient tissue samples to distinguish cancerous from healthy cells without the need for chemical staining.
CHARM is a pan-European collaboration between CRI, the University of Cambridge, Italian institutions Politecnico Di Milano and Consiglio Nazionale Delle Ricerche, the Jena University Hospital in Germany, and the firms INsociety from Italy and Inspiralia from Spain. The project coordinator is Dr Matteo Negro, CRI’s Chief Technology Officer.
CRI is to receive €1.3 million of the grant, which is being made to its wholly-owned Italian subsidiary Cambridge Raman Imaging s.r.l.
CHARM is one of the 42 projects selected for funding from 292 submitted in the first ever EIC Transition Challenges and among the only 9 selected in the framework of the “Medical Technology and Devices: from Lab to Patient” Challenge, intended to support moving technologies from laboratories into the real world. It aims to develop medical devices to the preclinical validation phase.
CRI’s Raman imaging technology uses graphene-based ultra-fast fibre lasers to generate digital images of patient tissue for automatic analysis by artificial intelligence to support diagnosis. Because the images are digital, they can be viewed remotely, allowing histopathologists to work more efficiently and to support regions and countries short of qualified staff. The technology also potentially opens the way for personalised treatments for cancer.
The EIC is Europe’s flagship innovation programme to identify, develop and scale up breakthrough technologies and game changing innovations.
Dr Matteo Negro, Cambridge Raman Imaging’s Chief Technology Officer and CHARM project coordinator, said:
“We are proud to see our technology recognised by the EU as a potentially disruptive innovation, able to strongly support histopathologists in their clinical routine, by providing objective chemical information on tissues to improve cancer diagnosis accuracy and personalised treatment selection.”
“CRI has a mission to bring coherent Raman histopathology to the clinical market and the EIC Transition grant will be boosting our activities towards a successful commercialisation of our innovation.”